Research

Photosynthesis is an important biochemical process in which plants, algae, and some bacteria harness the energy of solar light to produce food and oxygen that make up a large portion of the Earth's atmosphere. Photosynthesis starts with the capture of solar energy by light-harvesting complexes, which absorb and transfer this energy to reaction centers where the charge separation is generated. I exploit distinct biological, spectroscopic and microscopic methodologies to study the assembly, structure and interactions of photosynthetic macromolecular complexes. My long-term study will focus on the biogenesis, structure and function of bioenergetic membranes.
Redox-regulated distribution of respiratory complexes in cyanobacteria
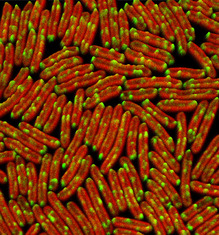
Using fluorescent tagging and live-cell confocal fluorescence microscopy imaging, we investigated the distribution of key respiratory electron donors, type-I NAD(P)H dehydrogenase (NDH-1) and succinate dehydrogenase (SDH), in live cells of the cyanobacterium Synechococcus elongatus PCC 7942. We found that, when cells are grown under low light, both complexes form discrete patches in the thylakoid membranes. Exposure to moderate light leads to redistribution of complexes, and they become evenly distributed in thylakoid membranes. The distribution of the complexes within the thylakoid membranes is under redox-regulated physiological control. Redistribution of the complexes correlates with a major change in the relative probability of the two electron transport pathways. Our results indicate that the sub-micron scale distribution of respiratory complexes governs the partitioning of reducing power and that redistribution of electron transport complexes on these scales is a physiological mechanism to regulate the pathways of electron flow.
Proc. Natl. Acad. Sci. USA, 2012, 109 (28): 11431-11436
Proc. Natl. Acad. Sci. USA, 2012, 109 (28): 11431-11436
Organization and interaction of the photosynthetic apparatus in purple photosynthetic bacteria
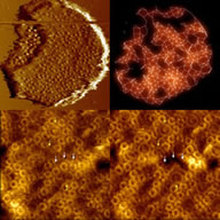
In photosynthetic organisms, membrane pigment-protein complexes (LH2, LH1) harvest solar energy and convert the sunlight into an electrical and redox potential gradient (reaction center) with high efficiency. I study the spatial organization of the bacterial photosynthetic apparatus, and the strategies employed for efficient harvesting and trapping of solar energy, as well as the long-distance quinone pathways (5). The first quantitative measurements of the unfolding process and structural stability of membrane proteins (LH2) in native biological membrane revealed forces and energies that assure structural and functional integrity of LH2, as well as how LH2 interact with other proteins in the supramolecular architecture. It suggests explicitly the importance of LH2 ring-shaped architecture, and that the complex stability is supported and depends on their specific molecular environment in native membrane. The acquired information gives hints how molecular interactions drive photosynthetic protein assembly.
Proc. Natl. Acad. Sci. USA, 2011, 108 (23): 9455-9459
J Struct Biol, 2011, 173 (1): 138-145
J Mol Biol, 2009, 393 (1): 27-35
Proc. Natl. Acad. Sci. USA, 2011, 108 (23): 9455-9459
J Struct Biol, 2011, 173 (1): 138-145
J Mol Biol, 2009, 393 (1): 27-35
Distribution and dynamics of light-harvesting complexes in red aglal photosynthetic membrane
The architecture of the entire photosynthetic membrane network determines, at the supramolecular level, the physiological roles of the photosynthetic protein complexes. I examine the supramolecular architectures of phycobilisomes (PBsomes) on the thylakoid membranes from the unicellular red alga Porphyridium cruentum using atomic force microscopy (AFM) (1) and electron microscopy (EM) (2). On the basis of the crowding organisation of PBsome on thylakoid membrane, I further investigate the diffusion dynamics of PBsomes in red algal cell using fluorescnece recovery after photobleaching (FRAP) (3). The mobility of PBsomes in cells were deeply estimated.
J Biol Chem, 2008, 283 (50): 34946-34953
PLoS ONE, 2009, 4 (4): e5295
Photosynth Res, 2008, 95 (2-3): 169-174
J Mol Biol, 2009, 393 (1): 27-35
J Biol Chem, 2008, 283 (50): 34946-34953
PLoS ONE, 2009, 4 (4): e5295
Photosynth Res, 2008, 95 (2-3): 169-174
J Mol Biol, 2009, 393 (1): 27-35
Single-molecule spectroscopy of light-harvesting complex
Photosynthetic organisms have developed multiple protective mechanisms to prevent photodamage in vivo under high-light conditions. I apply single-molecule spectroscopy to perform the first evaluation on the fluorescence dynamics of individual PBsomes of P. cruentum (4). The observations strongly indicate an energetic decoupling occurring in the light-harvesting complex and the photoprotection role of PBsomes to prevent photodamage of the photosynthetic reaction centers.
PLoS ONE, 2008, 3 (9): e3134
PLoS ONE, 2008, 3 (9): e3134
